Home
Lever Rule
Scheil
Back Diffusion
Articles
Working Group
Ursula
Kattner
Bill Boettinger
Dilip Banerjee
|
Lever rule solidification and Scheil
solidification are illustrated for the cases of two Sn-Bi-Pb alloys.
The thermodynamic description of Sn-Bi-Pb from Yoon and Lee,
Calphad 22 (1998) 167 was used for the
present calculations.
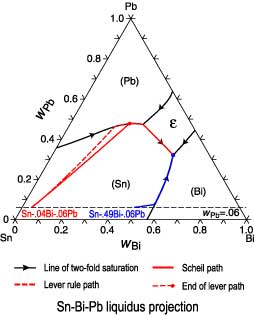
The liquid concentration paths for the alloys, Sn-.04Bi-.06Pb and
Sn-.49Bi-.06Pb, are shown together with the liquidus
projection of Sn-Bi-Pb system. The (Sn) phase is the primary
phase in all cases. Under Scheil conditions the
alloy Sn-.04Bi-.06Pb encounters the L -> (Sn) + (Pb) monovariant
eutectic where
both phases form simultaneously from the liquid phase. The path
encounters then the four phase reaction, L + (Pb) -> (Sn) +
epsilon.
Under Scheil assumptions fraction and concentration profile of the (Pb)
phase become fixed and solidification continues along the
monovariant eutectic, L -> (Sn) + epsilon, until solidification is
completed with
the ternary eutectic reaction, L -> (Sn) + epsilon + (Bi). The
alloy Sn-.49Bi-.06Pb encounters unter Scheil conditions the L ->
(Sn) + (Bi) monovariant eutectic. Both solid phases form
simultaneously from the liquid until the ternary eutectic L -> (Sn)
+ epsilon + (Bi) is encountered. The lever rule and Scheil paths
for this alloy are nearly identical.
|
|
| The sequence
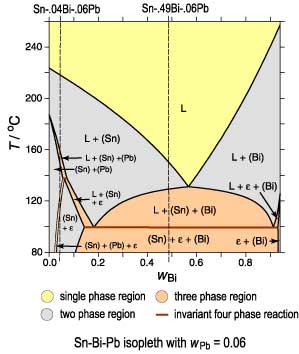
of phase formation during lever rule solidification can also be
obtained from an isopleth that includes the alloy composition.
However, since the tie-lines usually do not lie within the plane of the
isopleth, no information about phase amounts, phase composition or the
Scheil solidification can be obtained. |
 |
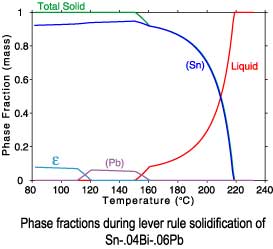
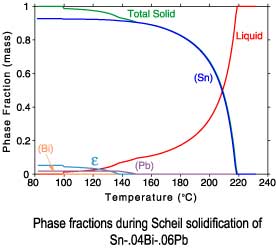
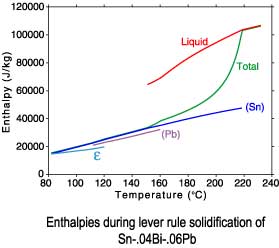
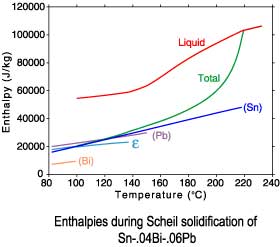
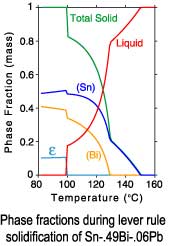
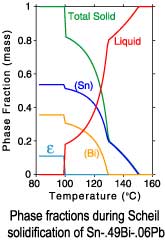
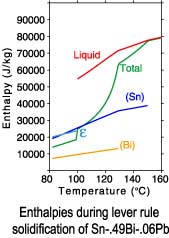
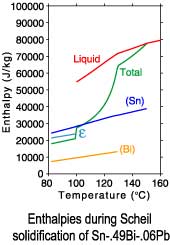
The differences between a lever rule and Scheil solidification paths can
be seen in plots of the phase fractions and enthalpies. Since under
Scheil assumptions the concentration of the solid phases is "frozen"
after solidification is complete, only the temperature dependence of
the enthalpies is recorded after completion of solidification.
Lever rule solidification
|
Scheil solidification
|
Sn-.04Bi-.06Pb
|
|
|
|
 |
 |
| Sn-.49Bi-.06Pb |
|
 |
 |
 |
 |
|

